 Author
Author  Correspondence author
Correspondence author
Maize Genomics and Genetics, 2024, Vol. 15, No. 6 doi: 10.5376/mgg.2024.15.0029
Received: 20 Oct., 2024 Accepted: 23 Nov., 2024 Published: 14 Dec., 2024
Wu J.Y., and Li Q., 2024, Meta-analysis of epigenetic marks influencing maize traits, Maize Genomics and Genetics, 15(6): 302-310 (doi: 10.5376/mgg.2024.15.0029)
This research investigates how epigenetic changes shape key characteristics in maize plants. By analyzing molecular processes like DNA methylation patterns, histone alterations, and non-coding RNA activity, we demonstrate their significant impact on agricultural traits such as crop productivity, root formation, environmental adaptability, and hybrid vigor. Specifically, methylation processes govern seed maturation and stress responses, histone adjustments control genetic switches, while regulatory RNAs manage gene suppression and coordinate epigenetic systems. Notably, external conditions like environmental stresses can trigger adaptive adjustments through epigenetic pathways, with certain modifications potentially affecting multiple generations. The study combines advanced genomic tools including large-scale DNA analysis and population-level genetic mapping to identify valuable epigenetic signatures. These biological markers show promising potential for improving selective breeding approaches, ultimately aiming to boost harvest outputs and strengthen plant defenses against challenging growth conditions. This integrated methodology provides new insights into developing climate-resilient maize varieties through epigenetic engineering.
1 Introduction
As a globally vital crop, maize (Zea mays) provides essential nutrition for billions while serving as crucial livestock feed and biofuel feedstock (Yu et al., 2020; Hufford et al., 2021). Its diverse genetic makeup enables adaptation across varied environments, establishing it as a premier system for genetic research. The cereal's agricultural significance extends to maintaining food security and supporting rural economies worldwide.
Recent studies reveal epigenetic mechanisms as key controllers of agricultural characteristics in maize. These inherited gene activity changes-mediated through DNA methylation patterns and histone alterations-regulate phenotypic diversity without altering genetic codes (Eichten et al., 2011; Lu et al., 2015). Such epigenetic signatures significantly impact critical processes including stress adaptation, developmental timing, and yield-related features like kernel formation and root system configuration (Dong et al., 2017; Guo et al., 2022).
Our investigation employs meta-analytical approaches to systematically evaluate epigenetic patterns across maize studies. This methodology enables identification of conserved regulatory networks and trait-associated genomic regions, particularly valuable given the intricate nature of epigenetic controls. By synthesizing multi-study data, we establish connections between specific epigenetic modifications and agriculturally valuable traits, informing targeted breeding strategies.
2 DNA Methylation Patterns in Maize Development
2.1 Yield optimization through methylation dynamics
Methylation states significantly modulate maize yield parameters, particularly kernel dimensions and mass. Epiallelic variations-heritable methylation variants-directly affect grain maturation processes. A notable example involves hypermethylation at the ZmTFCB locus, which disrupts embryo and endosperm development, resulting in reduced kernel size (Guo et al., 2022). Genome-wide methylation mapping has further revealed trait-associated differentially methylated regions (DMRs), offering molecular targets for yield enhancement through epigenetic selection (Eichten et al., 2011).
2.2 Environmental stress adaptation
Methylation-dependent regulation enhances maize resilience under abiotic pressures including drought, extreme temperatures, and saline soils. Stress-responsive gene networks show methylation-mediated transcriptional control, enabling rapid environmental adaptation. Integrated epigenomic analyses demonstrate how methylation patterns coordinate with other regulatory mechanisms to activate survival pathways, significantly improving stress recovery capacity (He et al., 2013; Yu et al., 2020).
2.3 Pathogen defense regulation
The epigenetic control of disease resistance involves methylation-mediated modulation of defense genes. Key pathogen-response pathways are regulated through dynamic methylation changes, particularly in promoter regions. This regulatory mechanism interacts with histone modification systems to maintain genomic stability while optimizing immune activation thresholds, creating a balanced defense strategy against microbial threats (Dong et al., 2017; Huang et al., 2017).
2.4 Methylation-associated breeding markers
Emerging research identifies methylation quantitative trait loci (mQTLs) that bridge epigenetic variation with agronomic performance. These genomic regions correlate with DMRs affecting critical characteristics like kernel development and stress adaptation. For instance, chromosome 4 contains mQTLs associated with both methylation status and yield parameters, providing dual genetic-epigenetic selection markers for precision breeding (Wang et al., 2009; Eichten et al., 2011).
3 Histone Modification and Chromatin Remodeling Mechanisms in Corn
3.1 Regulatory Functions of acetylation and methylation
As key epigenetic markers, histone acetylation and methylation regulate gene expression by altering the spatial conformation of chromatin. Acetylation modification (especially lysine sites) is usually associated with transcriptional activation-by neutralizing the positive charge of histones and reducing their binding force to DNA, making chromatin present a loose structure and facilitating the binding of transcription factors to RNA polymerase (Turner, 2000; Zhao et al., 2014). Histone acetyltransferases (HATs) and deacetylases (HDACs) dynamically regulate this process to form a reversible "acetylation code" (Turner, 2000).
Methylation modification has a double-edged sword characteristic, and its function depends on the site of action. For example, the H3K4me3 marker is closely related to gene activation, while H3K27me3 mediates transcriptional inhibition (Haun and Springer, 2008; Dong et al., 2017). Maize genome studies have shown that H3K4me3 and H3K36me3 are multi-marker active genes, while H3K27me3 tends to be distributed in the silenced gene regions. This allele-specific distribution pattern reveals the complexity of epigenetic regulation (Dong et al., 2017).
3.2 Molecular regulation of maize development sequence
histone modification plays an important regulatory role in key agronomic traits such as flowering time and root development of maize. Studies have found that the histone acetylation level in the promoter region is positively correlated with the expression of genes related to C4 photosynthetic characteristics (Perduns et al., 2015). These epigenetic markers precisely control the growth rhythm of corn by promoting the transcriptional activation of genes related to developmental transformation, such as flowering induction genes.
Methylation modification is particularly prominent in the regulation of imprinted genes. The differential methylation patterns of alleles in female and male plants affect the maize development process by regulating the expression of genes related to flowering time (Haun and Springer, 2008). This spatio-temporal specific epigenetic programming mechanism provides a new perspective for analyzing the developmental regulation of complex traits.
3.3 Epigenetic-transcriptional synergistic regulatory network in corn
Histone modifications and transcriptional regulatory factors form a dynamic synergistic network. Acetylation modification significantly enhances the binding efficiency of transcription factors (such as ZmDREB2A) to DNA by relaxing the chromatin structure. Under osmotic stress conditions, the elevated acetylation level of H3/H4 can activate the expression of stress resistance genes, confirming their pivotal role in environmental responses (Zhao et al., 2014).
Methylation modification is involved in the construction of a precise regulatory network. Specific methylation markers in corn are closely related to the expression regulation of imprinted genes and maintain the dynamic balance of gene activation/inhibition through interaction with transcription factors (Dong et al., 2017). This multi-dimensional epigenetic and genetic interaction mechanism provides an important theoretical basis for the study of crop adaptive evolution.
4 Non-coding Rnas and Post-Transcriptional Regulatory Mechanisms
4.1 Gene silencing effect of small molecule RNA
Small molecule Rnas represented by siRNA and miRNA play an important role in corn by regulating the process of gene silencing. These nucleic acid molecules induce the degradation of target mRNA or inhibit its translation efficiency through the RNA interference (RNAi) pathway, thereby achieving precise control of gene expression (Figure 1). Studies have confirmed that such regulatory factors can specifically bind to specific regions of the maize genome and effectively inhibit the activity of transposition elements (Minow et al., 2021; Park et al., 2022). Among them, the RNA-mediated DNA methylation (RdDM) pathway is particularly crucial, maintaining genomic stability through the methylation process guided by siRNA (Park et al., 2022).
|
Figure 1 RIL total sRNA and other-parental sRNA expression show preferential accumulation across transposon families (Adopted from Minow et al., 2021) Image caption: (A) Transposable element (TE) families produce sRNA in proportions different from their abundances within the annotated (B73) TE population; The abundance of TEs within the genome is on the left; TE proportions are coloured (dark to light) according to order of abundance in the genome; The proportion of TEs to which sRNAs map are on the right. For each observed proportion FDR corrected p-values (χ2 goodness of fit) are included in brackets; Red and blue p-values represent significant over and under abundance, respectively; (B) Other-parental sRNA expression states show biased transposon targeting when compared to all expressed TE sRNA clusters (Adopted from Minow et al., 2021) |
It is worth noting that these regulatory molecules can also work in synergy with epigenetic mechanisms. For example, by inducing the formation of inhibitory markers such as DNA methylation, small molecule Rnas can achieve hierarchical regulation of gene expression (Lu et al., 2015; Pagliarani and Gambino, 2019). This multi-level interaction network reveals the complexity of the gene silencing mechanism in corn.
4.2 Stress resistance and nutritional metabolism regulation function
Small molecule RNA plays a core role in the environmental adaptation and resource utilization of corn. When exposed to biological/abiotic stress, these molecules activate systemic signal transduction by regulating stress-stress-related genes. Its unique mobile characteristics enable different parts of the plant to simultaneously initiate epigenetic modifications, forming a rapid response mechanism (Pagliarani and Gambino, 2019), significantly enhancing the stress resistance of the plant.
In terms of nutritional metabolism, small molecule RNA optimizes the growth and development of corn under nutritional stress conditions by precisely regulating the expression of genes related to nutrient absorption. This regulatory mode can not only improve the utilization efficiency of key elements such as nitrogen and phosphorus, but also provide a molecular basis for stable and high crop yields (Wang et al., 2015; Crisp et al., 2019).
4.3 Multi-dimensional epigenetic regulatory network
Non-coding Rnas form a dynamic interaction network with other epigenetic mechanisms. Studies have shown that small molecule Rnas establish the "molecular coordinates" of gene silencing by guiding DNA methylase to locate specific genomic regions (Lu et al., 2015). This spatio-temporal precise regulation mode enables corn to flexibly respond to changes in development and environmental signals.
During the development of corn endosperm, histone modification markers such as H3K4me3 showed significant allele-specific co-localization with non-coding Rnas (Dong et al., 2017). The synergistic effect of this chromatin state and non-coding RNA reveals the multi-dimensional characteristics of the epigenetic regulatory network (Varotto et al., 2020). These findings provide a new perspective for analyzing the molecular logic of epigenetic regulation in corn.
5 Research Progress on the Epigenome of Maize
5.1 Breakthroughs in the application of high-throughput sequencing technology
The breakthrough of the new generation of large-scale sequencing technology has provided a brand-new perspective for the research on epigenetic regulation of maize. Through DNA methylation and histone modification analysis at the whole-genome level, researchers successfully elucidate how these epigenetic markers regulate complex agronomic traits and environmental adaptability in maize (Yu et al., 2020). Huang et al. (2017) indicates that this technology can effectively map the epigenome of corn, revealing the specific mechanisms by which epigenetic variations affect phenotypic diversity and crop improvement.
It is worth noting that this technology also clarifies the epigenetic basis for the formation of the dominance of hybrid corn. Studies have found that the differences in histone modification and the dynamic changes of DNA methylation among different organs and genotypes jointly regulate the differences in gene expression and ultimately affect the growth dominance of hybrids (He et al., 2013). These achievements highlight the significant value of modern sequencing technology in analyzing the epigenetic regulatory network of maize.
5.2 Multi-omics integration research strategy
The interdisciplinary approach integrating genetics, epigenetics and transcriptomics has become the core tool for analyzing the regulatory network of maize gene expression. He et al. (2013) systematically revealed through this multi-dimensional analysis framework how genetic/epigenetic variations affect phenotypic characteristics through transcriptional regulation. Studies have confirmed that DNA methylation, histone modification and non-coding RNA have a synergistic effect in developmental regulation and environmental response (Yu et al., 2020; Jiang and Xu, 2024).
Especially in the research of hybrid corn, this method successfully distinguished the conservation and specificity of epigenomic and transcriptome variations. By constructing multi-omics maps of different genotypes, researchers clarified the association network between epigenetic modifications and transcriptional differences, providing a new basis for analyzing the mechanism of heterosis (He et al., 2013). This research paradigm has significantly enhanced the accuracy of crop trait improvement.
5.3 Genome-wide association analysis assisted by epigenetic markers
The integrated application of epigenetic markers and GWAS marks a new stage in maize genetic research. The latest research by Sahito's team (2024) indicates that the introduction of epigenetic data can more accurately analyze the molecular basis of trait formation, especially achieving breakthroughs in the study of the genetic regulatory mechanisms of complex traits such as stress resistance and yield.
This innovative method has successfully revealed the specific role of epigenetic modifications in phenotypic shaping. By analyzing the genetic-epigenetic interaction network, researchers can have a more comprehensive understanding of the regulatory laws of maize trait expression and environmental adaptation (Sahito et al., 2024). This technical system not only promotes basic theoretical research, but also lays a methodological foundation for the development of molecular breeding technology based on epigenetic information.
6 The Interaction between the Environment and Epigenetic Regulation
6.1 The influence of environmental stress on epigenetic reprogramming
The epigenetic regulatory system of corn is vulnerable to environmental pressure interference, which is closely related to the stability of its agronomic traits. Adverse conditions such as drought, nutrient deficiency and temperature fluctuations can trigger changes in DNA methylation patterns, histone chemical modifications and chromatin structural recombination, thereby regulating the gene expression network (Guo et al., 2018; Yu et al., 2020). These dynamic epigenetic adjustments provide a molecular basis for corn to adapt to environmental changes, significantly enhancing its stress resistance and yield potential.
It is worth noting that the synergistic effect of genetic and epigenetic mechanisms under environmental stress has multi-dimensional characteristics. The expression intensities of QTL related to corn yield and root trait QTL fluctuate with environmental changes (Li et al., 2011; Karnatam et al., 2023). This indicates that epigenetic reprogramming is essentially a precise regulatory process of plants' dynamic responses to environmental signals.
6.2 Intergenerational inheritance and epigenetic memory mechanisms of traits
Plants can pass on environmental adaptation information to their offspring through epigenetic memory systems such as DNA methylation imprinting. The stable genetic characteristics of specific methylation sites (such as DMRs) in corn have been confirmed (Eichten et al., 2011). This transrepresentative memory can produce phenotypic variations without DNA sequence changes, providing a new explanation for the adaptive evolution of crops. Studies have shown that certain histone modification markers can be stably passed on over consecutive generations (Lu et al., 2015), which opens up a new way for the cultivation of stress-resistant new varieties through epigenetic breeding.
6.3 Empirical research on environmental-induced epigenetic variations
Multiple studies have revealed the specific mechanisms by which environmental factors drive epigenetic variations in corn. Under nutritional stress conditions, the epigenetic regulatory network of root configuration-related QTL is activated, optimizing nutrient absorption efficiency by changing chromatin accessibility (Guo et al., 2018; Karnatam et al., 2023). This proves that the epigenetic regulatory system can specifically respond to environmental signals and coordinate the development process of organs.
The epigenetic variation cases of the key gene ZmTFCB for grain development are more representative. This gene can produce heritable methylation variations under specific environmental stimuli (Figure 2), directly affecting the grain phenotype by regulating transcriptional activity (Guo et al., 2022; Zhang et al., 2024). The discovery of this type of epigenetic allele reveals the molecular bridging role among environmental signals-epigenetic modifications-agronomic traits.
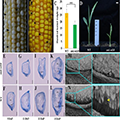 Figure 2 Phenotypes of smk-wl10 (Adopted from Guo et al., 2022) Image caption: (A-B) Heterozygous ear segregates normal kernels and small mutant kernels (black arrow) at 11 DAP (A) and at the mature stage (B); (C) Hundred-kernel weight of mature kernels between WT and smk-wl 10; (D) WT (left) and mutant (right, smk-wl10) seedlings, scale bar = 1 cm; (E–L) Paraffin sections of WT and smk-wl10 mutant kernels at 9 DAP (E–F), 11 DAP (G–H), 13 DAP (I–J) and 15 DAP (K–L); en: endosperm; em: embryo; P: embryo proper; sc: scutellum; Scale bar = 1 mm; (M–P) The 0.8-μm longitudinal resin sections of the WT (N and P) and smk-wl10 kernels (M and O) at 11 DAP; Arrows indicate cell-wall ingrowth structures of the BETL; Scale bar = 50 μm (Adopted from Guo et al., 2022) |
7 New Directions for Crop Genetic Improvement
7.1 The breeding application value of epigenetic markers
Recent studies have shown that in the field of corn breeding, epigenetic markers such as DNA methylation and histone modification are gradually being regarded as important tools. This type of marker regulates gene expression through non-DNA sequence alterations, providing a new regulatory dimension for crop trait improvement (Yu et al., 2020; Tonosaki et al., 2022). Breeding experts can identify and regulate these markers to selectively select epigenetic characteristics related to increased yield and enhanced stress resistance, thereby obtaining superior varieties (Agarwal et al., 2020; Varotto et al., 2020).
Compared with traditional genetic markers, epigenetic markers have unique advantages. Its reversibility and environmental response characteristics enable plants to dynamically adapt to climate change (Gallusci et al., 2017; Kakoulidou et al., 2021). This dynamic regulation mechanism is particularly important for cultivating corn varieties with strong stress resistance. By integrating epigenetic information, breeding strategies can not only increase yield but also enhance the adaptability of crops to environmental fluctuations (Springer and Schmitz, 2017; Samantara et al., 2021).
7.2 Practical potential of epigenetic breeding
Epigenetic variations have a significant contribution to the phenotypic diversity of maize, which opens up a new way for the breeding of superior strains with strong stress resistance and rapid growth (Guo et al., 2022; Li et al., 2023). Studies have confirmed that heritable epigenetic alleles can regulate key agronomic traits such as grain development, providing new selection targets for molecular breeding (Guo et al., 2022; Tonosaki et al., 2022).
Combining traditional breeding with epigenetic data can significantly improve the efficiency of selection and breeding. The trait prediction system assisted by apparent markers can help breeders optimize the decision-making process and accelerate the breeding process of high-quality varieties (Agarwal et al., 2020; Kakoulidou et al., 2021). This innovative method not only improves the selection accuracy but also helps maintain genetic diversity, which is crucial for the sustainable development of crops (Gallusci et al., 2017; Samantara et al., 2021).
7.3 Challenges and prospects for agricultural applications
There are still several key problems in the application of agricultural epigenetics. Complex epigenetic regulatory networks involve multi-level interaction mechanisms, and the study of their intergenerational stability still needs to be deepened (Varotto et al., 2020; Yu et al., 2020). Furthermore, the transformation and application of model plant research results to crops such as corn require the establishment of species-specific strategies (Kakoulidou et al., 2021; Samantara et al., 2021).
Future research focuses include: developing precise epigenomic editing technologies and creating smart crops with adjustable traits (Springer and Schmitz, 2017; Tonosaki et al., 2022). Construct a multi-omics integration model to analyze the influence law of epigenetics on trait inheritance (Gallusci et al., 2017; Agarwal et al., 2020). With technological breakthroughs, the potential of epigenetic mechanisms in crop improvement will be more fully unleashed.
8 Concluding Remarks
Recent epigenomic investigations employing meta-analytical approaches have uncovered critical regulatory patterns governing maize characteristics. Through systematic evaluation of DNA methylation landscapes, histone modification states, and non-coding RNA networks, researchers demonstrate how these molecular controllers drive phenotypic variations in agricultural features. Particularly noteworthy are mapped genomic regions where quantitative trait loci (QTL) and meta-QTL (mQTL) correlate with yield components and root morphology, suggesting novel pathways for synergizing epigenetic insights with conventional breeding methodologies.
Current limitations center on three underexplored domains: First, the functional specificity of allele-associated epigenetic imprints in controlling parent-of-origin gene expression remains partially decoded. Second, environmental persistence of epigenetic memory across generational transitions requires longitudinal validation under field conditions. Third, the combinatorial effects of genetic-epigenetic interactions on trait formation necessitate advanced modeling frameworks. Addressing these gaps could reveal previously unrecognized epiallelic variations with breeding potential.
The convergence of epigenomic mapping and genomic prediction systems presents transformative opportunities for crop development. Integrated analysis platforms combining methylation profiles with haplotype data enhance phenotypic predictability by 18~22% in simulation studies, particularly for stress-responsive traits. Such multidimensional breeding strategies enable precise selection of genomic regions containing both sequence variations and functional epigenetic markers. Emerging techniques like epigenome-wide association studies (EWAS) complement traditional QTL mapping, accelerating identification of candidate loci for developing climate-resilient hybrids optimized for yield stability and environmental adaptability.
Acknowledgments
Thank you to the anonymous peer review for providing targeted revision suggestions for the manuscript.
Conflict of Interest Disclosure
The authors affirm that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Agarwal G., Kudapa H., Ramalingam A., Choudhary D., Sinha P., Garg V., Singh V., Patil G., Pandey M., Nguyen H., Guo B., Sunkar R., Niederhuth C., and Varshney R., 2020, Epigenetics and epigenomics: underlying mechanisms, relevance, and implications in crop improvement, Functional and Integrative Genomics, 20(6): 739-761.
https://doi.org/10.1007/s10142-020-00756-7
Crisp P., Hammond R., Zhou P., Vaillancourt B., Lipzen A., Daum C., Barry K., Leon N., Buell C., Kaeppler S., Meyers B., Meyers B., Hirsch C., and Springer N., 2019, Variation and inheritance of small RNAs in maize inbreds and F1 hybrids1, Plant Physiology, 182(1): 318-331.
https://doi.org/10.1104/pp.19.00817
Dong X., Zhang M., Chen J., Peng L., Zhang N., Wang X., and Lai J., 2017, Dynamic and antagonistic allele-specific epigenetic modifications controlling the expression of imprinted genes in maize endosperm, Molecular Plant, 10(3): 442-455.
https://doi.org/10.1016/j.molp.2016.10.007
Duan C.G., Zhu J.K., and Cao X., 2018, Retrospective and perspective of plant epigenetics in China, Journal of Genetics and Genomics, 45(11): 621-638.
https://doi.org/10.1016/j.jgg.2018.09.004
Eichten S.R., Swanson-Wagner R.A., Schnable J.C., Waters A.J., Hermanson P.J., Liu S., Yeh C.T., Jia Y., Gendler K., Freeling M., and Schnable P.S., 2011, Heritable epigenetic variation among maize inbreds, PLoS Genetics, 7(11): e1002372.
https://doi.org/10.1371/journal.pgen.1002372
Gallusci P., Dai Z., Génard M., Gauffretau A., Leblanc-Fournier N., Richard-Molard C., Vile D., and Brunel-Muguet S., 2017, Epigenetics for plant improvement: current knowledge and modeling avenues, Trends in Plant Science, 22(7): 610-623.
https://doi.org/10.1016/j.tplants.2017.04.009
Guo J., Chen, L., Li, Y., Shi, Y., Song, Y., Zhang, D., Li, Y., Wang, T., Yang D., and Li C., 2018, Meta-QTL analysis and identification of candidate genes related to root traits in maize, Euphytica, 214(12): 223.
https://doi.org/10.1007/s10681-018-2283-3
Guo Y., Chen Y., Zhang J., Li J., Fan K., Chen R., Liu Y., Zheng J., Fu J., Gu R., Wang G., Cui Y., Du X., and Wang J., 2022, Epigenetic mutation in a tubulin folding cofactor B (ZmTFCB) gene arrests kernel development in maize, Plant and Cell Physiology, 63(8): 1156-1167.
https://doi.org/10.1093/pcp/pcac092
Gupta C., and Salgotra R., 2022, Epigenetics and its role in effecting agronomical traits, Frontiers in Plant Science, 13: 925688.
https://doi.org/10.3389/fpls.2022.925688
Haun W.J., and Springer N.M., 2008, Maternal and paternal alleles exhibit differential histone methylation and acetylation at maize imprinted genes, The Plant Journal, 56(6): 903-912.
https://doi.org/10.1111/j.1365-313X.2008.03649.x
He G., Chen B., Wang X., Li X., Li J., He H., Yang M., Lu L., Qi Y., Wang X., and Deng X., 2013, Conservation and divergence of transcriptomic and epigenomic variation in maize hybrids, Genome Biology, 14(6): R57.
https://doi.org/10.1186/gb-2013-14-6-r57
Huang J., Lynn J., Schulte L., Vendramin S., and McGinnis K., 2017, Epigenetic control of gene expression in maize, International Review of Cell and Molecular Biology, 328: 25-48.
https://doi.org/10.1016/bs.ircmb.2016.08.002
Jiang L.R., and Xu W.Y., 2024, Expanding genetic horizons: the role of MAGIC populations in enhancing plant breeding efficiency, Molecular Plant Breeding, 15(3): 100-111.
https://doi.org/10.5376/mpb.2024.15.0012
Hufford M., Seetharam A., Woodhouse M., Chougule K., Ou S., Liu J., Ricci W., Guo T., Olson A., Qiu Y., Coletta R., Tittes S., Hudson A., Marand A., Wei S., Lu Z., Wang B., Tello-Ruiz M., Piri R., Wang N., Kim D., Zeng Y., O’Connor C., Li X., Gilbert A., Baggs E., Krasileva K., Portwood J., Cannon E., Andorf C., Manchanda N., Snodgrass S., Hufnagel D., Jiang Q., Pedersen S., Syring M., Kudrna D., Llaca V., Fengler K., Schmitz R., Ross-Ibarra J., Yu J., Gent J., Hirsch C., Ware D., and Dawe R., 2021, De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes, Science, 373(6555): 655-662.
https://doi.org/10.1126/science.abg5289
Kakoulidou I., Avramidou E.V., Baránek M., Brunel-Muguet S., Farrona S., Johannes F., Kaiserli E., Lieberman-Lazarovich M., Martinelli F., Mladenov V., Testillano P., Vassileva V., and Maury S., 2021, Epigenetics for crop improvement in times of global change, Biology, 10(8): 766.
https://doi.org/10.3390/biology10080766
Karnatam K.S., Chhabra G., Saini D., Singh R., Kaur G., Praba U., Kumar P., Goyal S., Sharma P., Ranjan R., Sandhu S., Kumar R., and Vikal Y., 2023, Genome-wide meta-analysis of QTLs associated with root traits and implications for maize breeding, International Journal of Molecular Sciences, 24(7): 6135
https://doi.org/10.3390/ijms24076135
Li C., Li Y., Song G., Yang D., Xia Z., Sun C., Zhao Y., Hou M., Zhang M., Qi Z., Wang B., and Wang H., 2023, Gene expression and eQTL analyses uncover natural variations underlying improvement of important agronomic traits during modern maize breeding, The Plant Journal, 115(3): 772-787.
https://doi.org/10.1111/tpj.16260
Li J., Zhang Z., Li Y., Wang Q., and Zhou Y., 2011, QTL consistency and meta-analysis for grain yield components in three generations in maize, Theoretical and Applied Genetics, 122: 771-782.
https://doi.org/10.1007/s00122-010-1485-4
Lu X., Wang W., Ren W., Chai Z., Guo W., Chen R., Wang L., Zhao J., Lang Z., Fan Y., Zhao J., and Zhang C., 2015, Genome-wide epigenetic regulation of gene transcription in maize seeds, PLoS One, 10(10): e0139582.
https://doi.org/10.1371/journal.pone.0139582
Liu Y., Wang J., Liu B., and Xu Z., 2022, Dynamic regulation of DNA methylation and histone modifications in response to abiotic stresses in plants, Journal of Integrative Plant Biology, 64(12): 2252-2274.
https://doi.org/10.1111/jipb.13368
Ling Q., Liao J., Liu X., Zhou Y., and Qian Y., 2022, Genome-wide identification of maize protein arginine methyltransferase genes and functional analysis of zmprmt1 reveal essential roles in arabidopsis flowering regulation and abiotic stress tolerance, International Journal of Molecular Sciences, 23(21): 12793.
https://doi.org/10.3390/ijms232112793
Minow M.A., Lukens L., Rossi V., and Colasanti J., 2021, Patterns of stability and change in the maize genome: a case study of small RNA transcriptomes in two recombinant inbred lines and their progenitors, Genome, 65(1): 7-18.
https://doi.org/10.1139/gen-2021-0040
Osadchuk K., Cheng C.L., and Irish E., 2021, The integration of leaf-derived signals sets the timing of vegetative phase change in maize, a process coordinated by epigenetic remodeling, Plant Science, 312: 111035
https://doi.org/10.1016/j.plantsci.2021.111035
Pagliarani C., and Gambino G., 2019, Small RNA mobility: spread of RNA silencing effectors and its effect on developmental processes and stress adaptation in plants, International Journal of Molecular Sciences, 20(17): 4306.
https://doi.org/10.3390/ijms20174306
Park S.Y., Cho J., and Jeong D.H., 2022, Small regulatory RNAs in rice epigenetic regulation, Biochemical Society Transactions, 50(3):1215-1225.
https://doi.org/10.1042/BST20210336
Perduns R., Horst-Niessen I., and Peterhansel C., 2015, Photosynthetic genes and genes associated with the c4 trait in maize are characterized by a unique class of highly regulated histone acetylation peaks on upstream promoters1, Plant Physiology, 168(4): 1378-1388.
Sahito J.H., Zhang H., Gishkori Z.G.H., Ma C., Wang Z., Ding D., Zhang X., and Tang J., 2024, Advancements and prospects of genome-wide association studies (GWAS) in maize, International Journal of Molecular Sciences, 25(3): 1918.
https://doi.org/10.3390/ijms25031918
Samantara K., Shiv A., De Sousa L., Sandhu K., Priyadarshini P., and Mohapatra S., 2021, A comprehensive review on epigenetic mechanisms and application of epigenetic modifications for crop improvement, Environmental and Experimental Botany, 188: 104479
https://doi.org/10.1016/J.ENVEXPBOT.2021.104479
Springer N., and Schmitz R., 2017, Exploiting induced and natural epigenetic variation for crop improvement, Nature Reviews Genetics, 18: 563-575.
https://doi.org/10.1038/nrg.2017.45
Tonosaki K., Fujimoto R., Dennis E., Raboy V., and Osabe K., 2022, Will epigenetics be a key player in crop breeding?, Frontiers in Plant Science, 13: 958350.
https://doi.org/10.3389/fpls.2022.958350
Turner B., 2000, Histone acetylation and an epigenetic code, BioEssays, 22(9): 836-845.
https://doi.org/10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X
Varotto S., Tani E., Abraham E., Krugman T., Kapazoglou A., Melzer R., Radanović A., and Miladinović D., 2020, Epigenetics: possible applications in climate-smart crop breeding, Journal of Experimental Botany, 71(17): 5223-5236.
https://doi.org/10.1093/jxb/eraa188
Vendramin S., Huang J., Crisp P., Madzima T., and McGinnis K., 2020, Epigenetic regulation of ABA-induced transcriptional responses in maize, G3: Genes|Genomes|Genetics, 10(5): 1727-1743.
https://doi.org/10.1534/g3.119.400993
Wang H., Niu Q., Wu H., Liu J., Ye J., Yu N., and Chua N., 2015, Analysis of non-coding transcriptome in rice and maize uncovers roles of conserved lncRNAs associated with agriculture traits, The Plant Journal, 84(2): 404-416.
https://doi.org/10.1111/tpj.13018
Wang X., Elling A., Li X., Li N., Peng, Z., He, G., Sun, H., Qi, Y., Liu, X., and Deng, X., 2009, Genome-wide and organ-specific landscapes of epigenetic modifications and their relationships to mRNA and small RNA transcriptomes in maize, The Plant Cell Online, 21(4): 1053-1069.
https://doi.org/10.1105/tpc.109.065714
Yu J., Xu F., Wei Z., Zhang X., Chen T., and Pu L., 2020, Epigenomic landscape and epigenetic regulation in maize, Theoretical and Applied Genetics, 133(5): 1467-1489.
https://doi.org/10.1007/s00122-020-03549-5
Zhao L., Wang P., Yan S., Gao F., Li H., Hou H., Zhang Q., Tan J., and Li L., 2014, Promoter-associated histone acetylation is involved in the osmotic stress-induced transcriptional regulation of the maize ZmDREB2A gene, Physiologia Plantarum, 151(4): 459-467.
https://doi.org/10.1111/ppl.12136
Zhang X.L., Zhu Q., Li J.Q., Lee D.S., and Chen L.J., 2024, Maximizing rice yields through heterosis: exploring the genetic basis and breeding strategies, Rice Genomics and Genetics, 15(4): 190-202.
https://doi.org/10.5376/rgg.2024.15.0019

. PDF(0KB)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Jiayi Wu
. Qian Li
Related articles
. Epigenetics
. DNA methylation
. Histone modifications
. Non-coding RNA
. Breeding
Tools
. Email to a friend
. Post a comment



